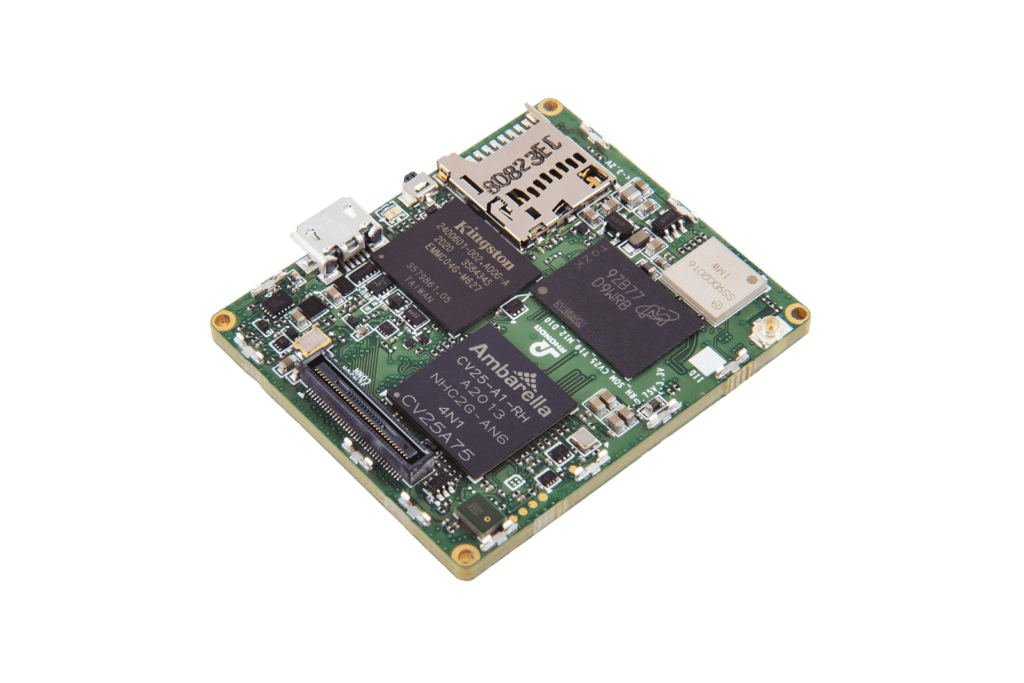
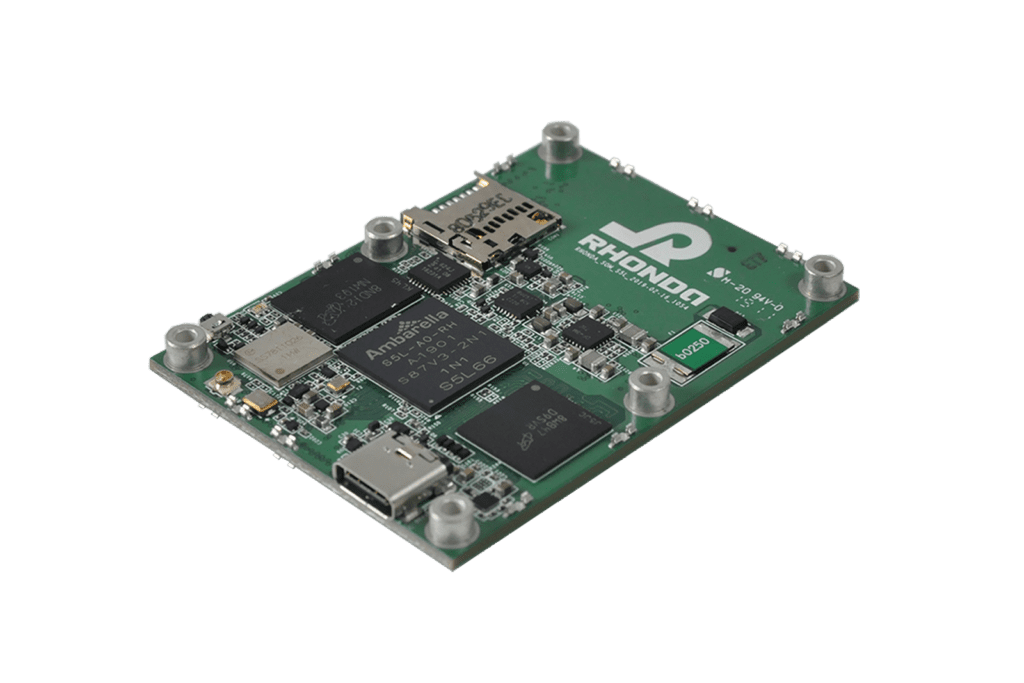
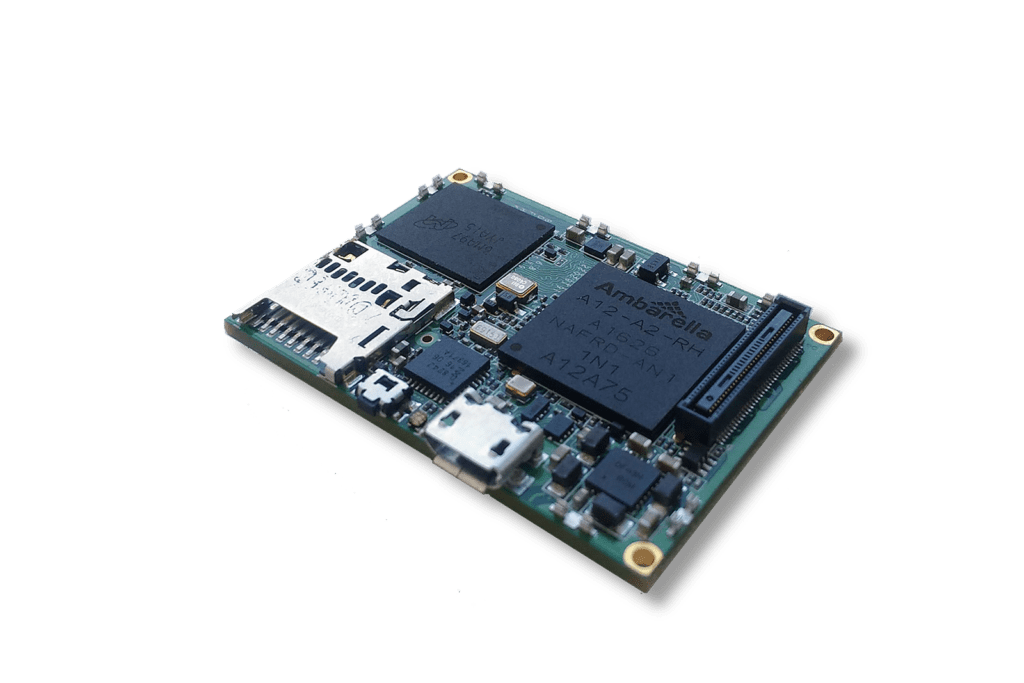
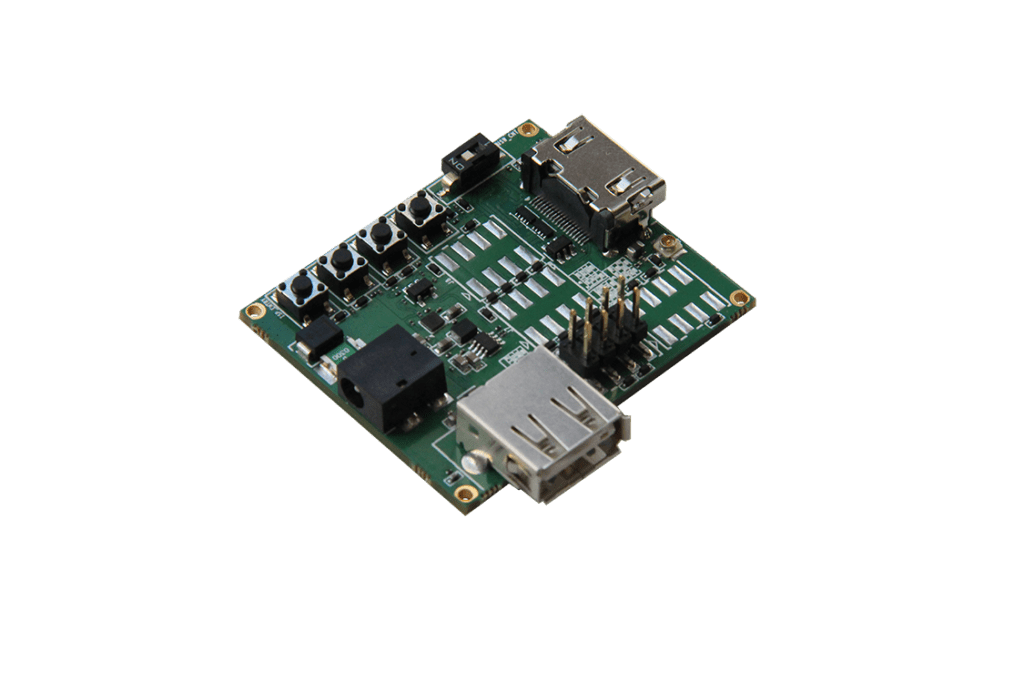
New
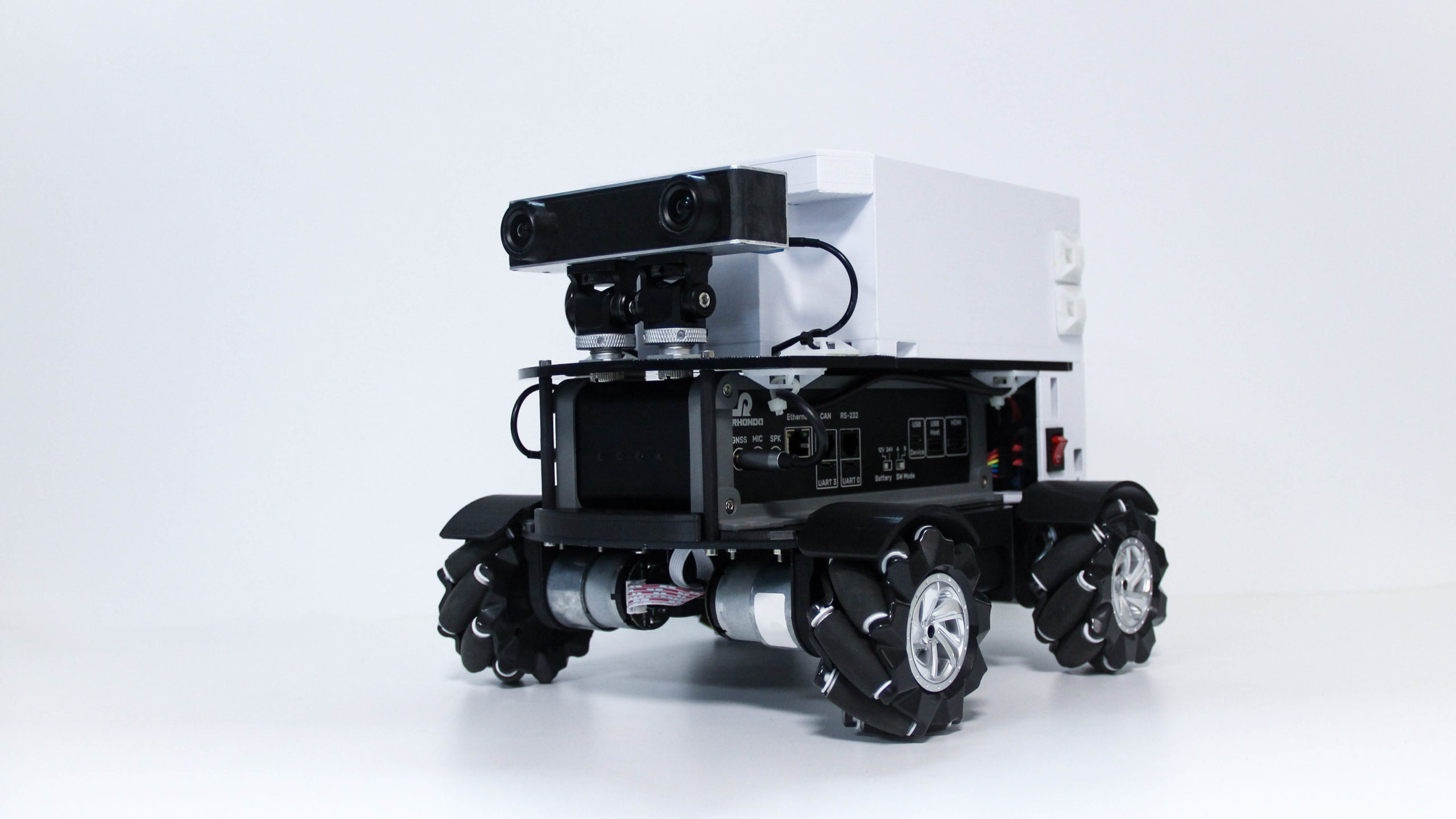
GMSL2 CAMERA EVK
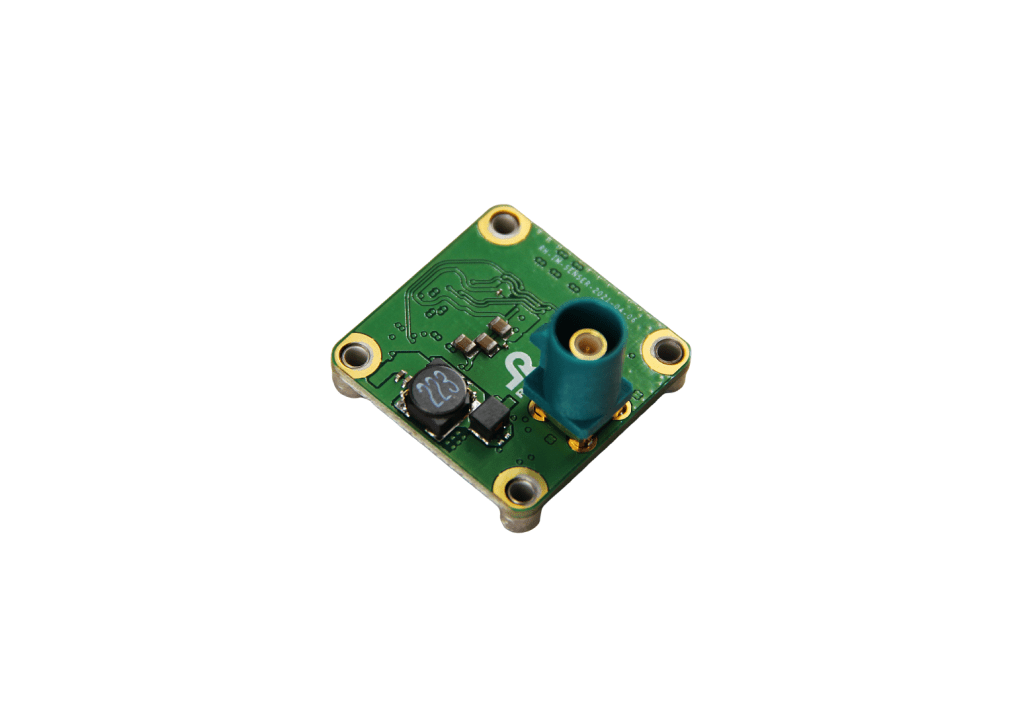
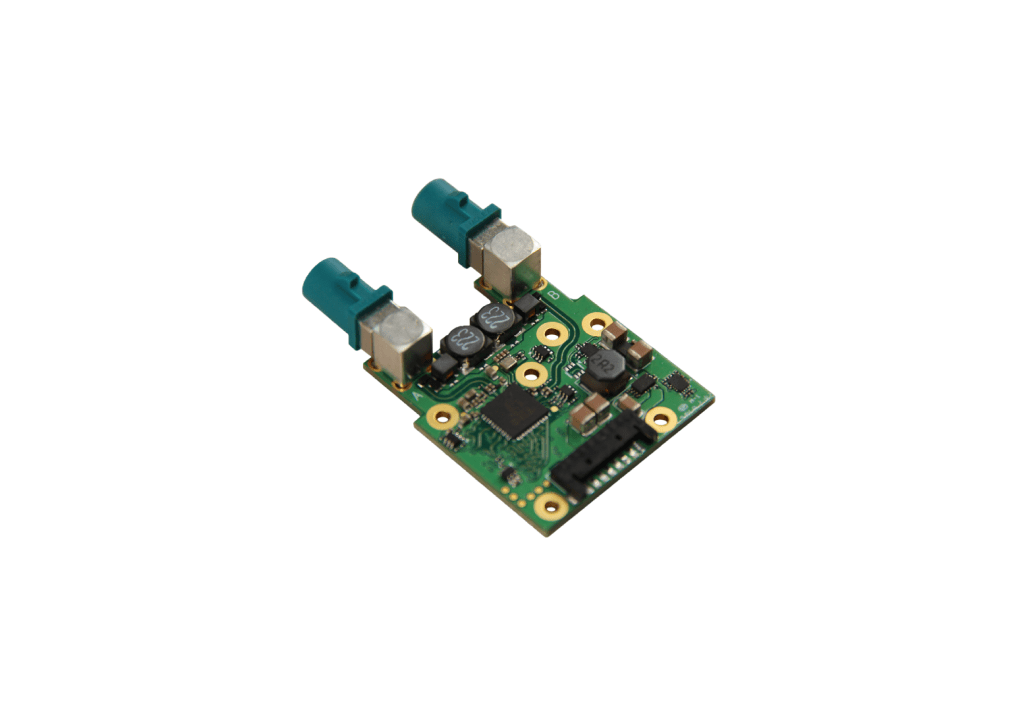
Camera Hardware Design
Rhonda creates innovative camera designs with the best camera processor (SoC) technologies, image sensor and other hardware components
Rhonda 快速168体彩网数据 Software is a close Ambarella's design partner with many years of experience in the Imaging business, providing camera solutions based on A12S, A12W, A9SE, H2, H22, S2L, S3L, S5L, CV2, CV22
The Rhonda 快速168体彩网数据 System on a Module (SoM) platform can accelerate your camera project or prototype. SoM is a PCB-level camera module with state-of-the-art Rhonda 快速168体彩网数据 Image Quality and a pre-defined feature set
Rhonda 快速168体彩网数据 is a one-stop shop for camera schematics and high-frequency HDI PCB layouts with interdisciplinary coordination with ME, optics and thermal design
The best Ambarella silicon in your product
Camera Software Development
Breathing life into bare metal
Highly customizable, continuously evolving Cross-platform middleware accelerates camera software development
Synchronized video capture and balanced image quality auto-correction on multi-sensor / multi-SoC cameras
Real-time video stitching for dual sensor cameras with back-to-back lens alignment
10+ Years of deep learning neural network porting and acceleration to execute complex computer vision features on-camera
Rhonda's 新五分钟彩结果直播实时更新 幸运官方飞行艇168结果号查询: 2-way camera connection, archived storage, event notifications, video transcoding & streaming
Android and iOS applications for camera setup, live video streaming and over-the-air firmware updates
Computer Vision On The Edge
Ambarella CVFlow power efficient & low latency DNN inference engine and our services of making Neural Nets run on cameras
The Rhonda 快速168体彩网数据 Software Computer Vision Team is capable of training new Neural Networks from scratch, and combining classical CV methods (such as tracking) with multiple detection and recognition DNNs into the whole system to solve customers’ unique needs | If an Ambarella-optimized DNN library is supplied by a CV partner, we can provide integration services: configure the AI library to meet quality/speed goals, enable input video at a specific resolution and FPS, implement DNN results post-processing, and delivery to external systems and decision-making |
When it comes to porting customers’ or open source AI onto the Ambarella CVflow, Rhonda’s CV Team applies its platform-specific knowledge to convert Pytorch/Tensorflow CNN/RNN into hardware accelerated representation: replacing the heavy backbone with a mobile-friendly one, substituting unsupported operations, splitting big networks into multiple modules running on the CVflow accelerator or ARM processor |
As an integral part of each CV project, On-chip validation environment and methodology allows Rhonda 快速168体彩网数据 Software to measure accelerated DNN performance during the different stages of a project cycle | To improve DNN inference performance on the edge, Rhonda 快速168体彩网数据 Software is ready to apply its extensive experience in DNN architecture optimization, pruning and quantization-aware re-training |
新五分钟彩结果直播实时更新 幸运官方飞行艇168结果号查询
Celesta – a ready-to-deploy camera-connected Video Cloud for centralized video archiving and data storage
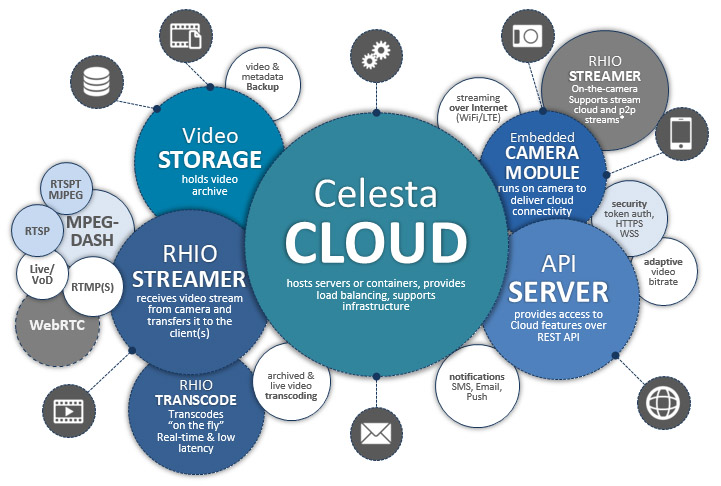
Deployment available both on bare metal servers and on Google Kubernetes Engine (GKE) clusters
Video records and Camera events with JPEG thumbnails. Metadata from Camera-connected sensors
Live stream from Camera or Video on Demand (VOD) from Storage: over HTTP in MPEG-DASH, RTMP and RTSP using Android / iOS SDK, MJPEG on any platform. Low latency transcoding of VOD & Live stream
REST API (JSON) with 200+ HTTP REST API resources: Multi-tenant business model, External Billing support over REST, JSON; WebSocket and Webhooks for notifications and subscriptions
Cross platform Embedded Camera Module: Windows / Linux / Embedded Linux with Real-time communication protocol using WebSocket Secure transport
Seeing Is Believing
A picture is worth a thousand words... What about a video?

Ambarella CV25 SoC-based Dual Camera Platform with integrated ADAS & DMS
This Platform is capable of analyzing both on-the-road situations and driver attention, as well as issuing warnings and capturing corresponding video events remotely on Celesta - Rhonda 快速168体彩网数据 Software's video cloud service. Cloud-based Web UI and Mobile App allow users to manage the connected cameras, and review on-the-road events gathered along with location data.
The Rhonda 快速168体彩网数据 Software Fleet Cam Proto is ready for use and testing. It provides a solid basis for the development of customized Driver Safety Systems, that are in high demand in markets such as:
· Commercial & Passenger Transport Fleet Management
· Telematics Service Providers
· Telematics Insurance
· Car-Sharing

Person Re-Identification on the Camera Edge
The demo setup is based on Rhonda 快速168体彩网数据 Software CV2 System on a Module (SoM) and allows to learn and re-identify person in the live camera feed. Re-Identification algorithm is sophisticated enough to do body tracking and appearance model re-learning on the go, when conditions allows. Identity models could be shared to other cameras for multi-camera person tracking.
To demonstrate the power of DNN acceleration of the Ambarella CV2 platform in addition to re-ID, the Pose Estimation and Activity Recognition nets where running on each person in the camera field of view.
Camera Engineering Services by Rhonda 快速168体彩网数据 Software
We have extensive expertise in
- Still and Video processing,
- Connected devices,
- Computer Vision,
- Cloud based services,
- Mobile Applications, etc.

Camera Systems on Modules by Rhonda 快速168体彩网数据 Software
- Rapid Proof of Concept with ready evaluation kits
- Custom Prototypes to study user experience and proof the market
- Compact camera boards for faster time-to-market with low to mid volume products.
The variety of SOM designs is available to address your project needs.
Image Quality Tuning
Build an optical system most appropriate for Your application
Optical system design
Image sensor & lens selection for your product needs
Adequate equipment
Image Quality (IQ) Lab for thorough Optical system analysis
Skilled experts
IQ experts configure the Ambarella SoC IQ Pipeline to deliver the best Camera IQ possible. Fully customizable IQ tuning for Computer vision applications
Baseline IQ tuning
Rhonda's 3A (AF, AE, AWB) algorithms, Noise reduction, Tone mapping, Color fidelity adjustment, Aberrations and shading compensation, Distortion correction, Image sharpening, Local contrast enhancement, and more
Manufacturing Control
Make Your camera factory process reliable and manageable
Manufacturing Quality Control
Automated testing with quantitative pass/fail criteria to find and filter out malfunctioning components at different assembly stages
Incoming Optical modules testing
Dust contamination checks, lens alignment verification, focus uniformity verification
Per-unit Factory IQ Calibration
Defective pixel correction, white balancing, lens shading compensation, etc.
Computer vision validation algorithms
Objective and consistent decision-making procedures unbiased by human-factor
Camera Development Options
For camera solution feasibility
For rapid camera prototyping
For 幸运澳洲5历史记录 camera development
About Rhonda 快速168体彩网数据 Software
Just a few highlights to tell our story
Our Partners
We have close relationships with the most famous names in the camera manufacturing world
 |  |  |  |
 |  |  |  |
 |  |  |  |
 |  |  |
Contact新的168飞艇直播结果速看
Let us know how we can help you